
Development of a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus, the Principal Global Agent of Mucormycosis in Humans
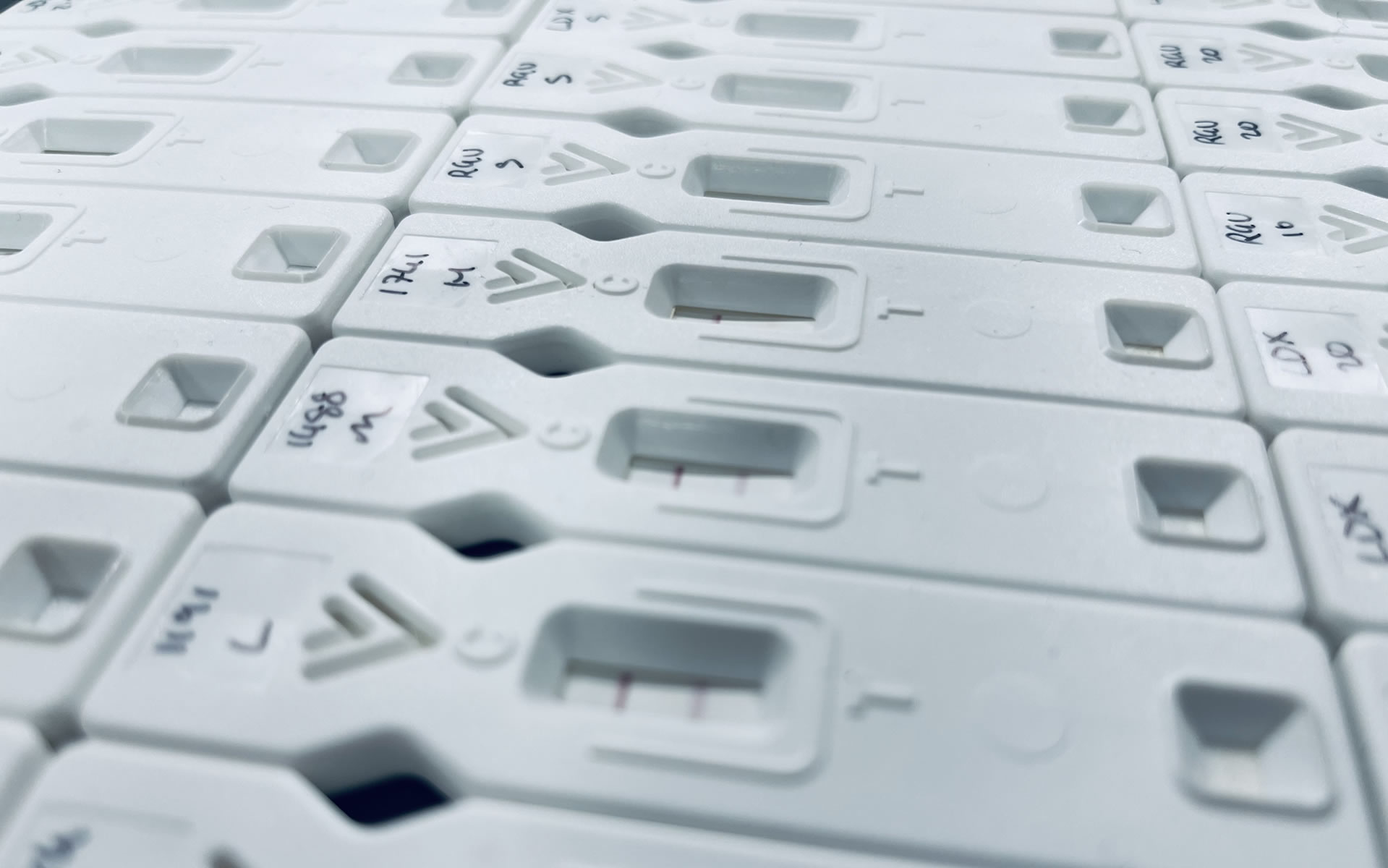

This is the first time that a quick and sensitive lateral-flow test for Mucormycosis has been reported, providing a point-of-care test for the disease.
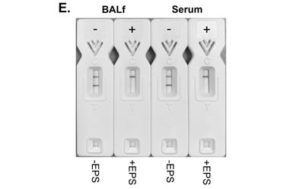
Using purified EPS from human-pathogenic mucoralean fungi, the LFD was shown to be species-specific, detecting Rhizopus arrhizus (syn. R. oryzae) only
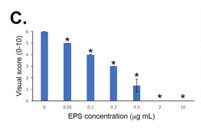
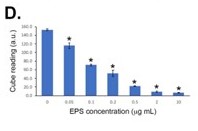
The sensitivity of the LFD was determined using EPS from R. arrhizus var. arrhizus (CBS112.07) diluted into the running buffer. Using both a score card and a Cube reader there were sequential and significant decreases in test (T) line intensities with increases in EPS concentrations between 0 μg EPS/mL (running buffer only) and 10 μg EPS/mL. Based on these results, the analytical limit of detection (LOD) was shown to be ~50 ng EPS/mL for the running buffer, using both scoring systems.


Lateral Dx
Unit 1, Block 7,
Cooperage Way, Alloa,
FK10 3LP, UK
01259 793041
Copyright © Lateral Dx 2022
Website Design by activ Digital Marketing
Stay informed on the latest lateral flow test development and manufacture industry news, rapid antibody screening developments, our sample antibody conjugation projects and everything in between. Fill in the form below to start receiving our newsletter.